24-hour hotline:+8613662168047
Keyword search: battery plant , lithium battery factory , power bank works , lifepo4 battery mill , Pallet Trucks LiFePO4 Battery, LiFePO4 Pallet Trucks Battery, Lithium Pallet Trucks Battery,
The development of negative electrode materials is characterized by high specific capacity, high charge and discharge efficiency, high cycling performance, and lower cost. 1 Carbon negative electrode material 1.1 Graphite graphite material has good conductivity, high crystallinity, and a good layered structure, suitable for lithium insertion and extraction, forming a lithium graphite interlayer compound Li GIC. The charge discharge specific capacity can reach over 300mAh/g, the charge discharge efficiency is over 90%, and the irreversible capacity is less than 50mAh/g. Machill et al. [21-22] can improve the cycling performance of AI electrodes by adding some metal elements that are soluble in Al or can form intermetallic compounds with Al, such as Ni, Cu, Mg, etc., to improve the diffusion rate of Li during the embedding process in the negative electrode, thereby enhancing the cycling performance of A1 electrodes.
The negative electrode of a lithium-ion battery is made by mixing the negative electrode active material carbon material or non carbon material, binder, and additives into a paste like adhesive, which is evenly applied to both sides of the copper foil, dried, and rolled. The key to the successful production of lithium-ion batteries lies in the ability to prepare negative electrode materials that can reversibly remove/insert lithium ions. Generally speaking, choosing a good negative electrode material should follow the following principles: high specific energy; The electrode potential relative to the lithium electrode is low; Good reversibility of charge and discharge reactions; Good compatibility with electrolytes and binders; Small specific surface area (<10m2/g), high true density (>2.0g/cm3); Good size and mechanical stability during lithium insertion process; Abundant resources and low prices; Stable and non-toxic in the air. At present, the negative electrode materials commonly used in lithium-ion batteries are generally carbon materials, such as graphite, soft carbon (such as coke), hard carbon, etc. The negative electrode materials being explored include nitrides, PAS, tin based oxides, tin alloys, nano negative electrode materials, and other intermetallic compounds. The article briefly introduces the current research status of practical carbon materials and non carbon materials being explored. 1 Carbon negative electrode material 1.1 Graphite graphite material has good conductivity, high crystallinity, and a good layered structure, suitable for lithium insertion and extraction, forming a lithium graphite interlayer compound Li GIC. The charge discharge specific capacity can reach over 300mAh/g, the charge discharge efficiency is over 90%, and the irreversible capacity is less than 50mAh/g. The deintercalation reaction of lithium in graphite occurs at around 0-0.25V (vs. Li+/Li), with a good charging and discharging potential platform, which can be matched with positive electrode materials such as LiCoO2, LiNiO2, LiMn2O4 that provide lithium sources. The resulting battery has a high average output voltage and is currently the most widely used negative electrode material in lithium-ion batteries. Graphite includes two categories: artificial graphite and natural graphite. Artificial graphite is produced by high-temperature graphitization treatment of easily graphitized carbon (such as asphalt coke) in N2 atmosphere at 1900-2800 ℃. Common artificial graphite includes mesocarbon microspheres (MCMB) and graphite fibers. There are two types of natural graphite: amorphous graphite and flake graphite. The purity of amorphous graphite is low, and the interplanar spacing (d002) of graphite is 0.336nm. The main structure is the 2H crystal plane sorting, arranged in ABAB order, with a reversible specific capacity of only 260mAh/g and an irreversible specific capacity of over 100mAh/g. The interplanar spacing (d002) of flake graphite is 0.335nm, mainly consisting of a 2H+3R crystal plane ordering structure, where the graphite layers are arranged in two orders: ABAB... and ABCABC. Scale graphite containing over 99% carbon has a reversible capacity of 300-350mAh/g. Due to the graphite spacing (d002=0.34nm) being smaller than the interplanar spacing (d002=0.37nm) of the lithium interlayer compound Li GIC, the graphite spacing changes during charge and discharge processes, which can easily cause graphite layer peeling and pulverization, as well as lithium and organic solvent co embedding in the graphite layer and organic solvent decomposition, which will affect the cycling performance of the battery. Therefore, people have also studied other graphite materials, such as modified graphite and graphitized carbon fibers. 1.2 Soft carbon, also known as easily graphitized carbon, refers to amorphous carbon that can be graphitized at high temperatures above 2500 ℃. The crystallinity (i.e. graphitization degree) of soft carbon is low, the grain size is small, the interplanar spacing (d002) is large, and it has good compatibility with the electrolyte. However, the irreversible capacity of the first charge discharge is high, the output voltage is low, and there is no obvious charge discharge plateau potential. Common soft carbons include petroleum coke, needle coke, carbon fiber, carbon microspheres, etc. 1.3 Hard carbon refers to carbon that is difficult to graphitize, which is the pyrolytic carbon of polymer materials. This type of carbon is also difficult to graphitize at high temperatures above 2500 ℃. Common hard carbons include resin carbon (such as phenolic resin, epoxy resin, polyfurfuryl alcohol PFA-C, etc.), organic polymer pyrolytic carbon (PVA, PVC, PVDF, PAN, etc.), and carbon black (acetylene black). Among them, polyfuryl alcohol resin carbon PFA-C has been used as a negative electrode material for lithium-ion batteries by Sony Corporation in Japan. The capacity of PFA-C can reach 400mAh/g, and the interplanar spacing (d002) of PFA-C is comparable, which is beneficial for lithium insertion without causing significant structural expansion, and has good charge discharge cycling performance. Another type of hard carbon material is polyacene (PAS), an amorphous semiconductor material obtained by pyrolysis of phenolic resin below 800 ℃. Its capacity is as high as 800mAh/g, and the interplanar spacing is 0.37-0.40nm, which is conducive to lithium insertion and deintercalation and has good cycling performance. 2 Non carbon negative electrode materials 2.1 Nitride lithium carbon materials have good rechargeable performance, small volume change when lithium is embedded, and good safety performance. They are a good negative electrode material and have been widely used in industry. However, their specific capacity is low (LiC6 is 372mAh/g), and the disintegration of carbon materials can cause capacity decay. Therefore, people are trying to find other non carbon negative electrode materials to replace carbon negative electrode materials, in order to solve this problem. In recent years, many researchers have studied nitride systems. The synthesis of nitrides can be traced back to the 1940s and 1950s in Germany's R Juza et al. conducted research on the synthesis and structure of this; In the 1980s, there were many studies on Li3N as a solid electrolyte. Li3N has good ion conductivity, but its decomposition voltage is very low (0.44V), so it is obviously not suitable as an electrode material directly. Transition metal nitrides have good chemical stability and electronic conductivity, while lithium transition metal nitrides possess both properties and should be suitable as electrode materials. Nitrided compounds belong to the anti fluorite or Li3N structure, with good ion conductivity (Li3N conductivity is 10-3S226; cm-1) and electrode potential close to metallic lithium, which may be used as negative electrodes for lithium-ion batteries. At present, the nitrogen doped materials studied include Li7MnN4 and Li3FeN2, which belong to the anti fluorite structure, and Li3-xCoxNoLi7MnN4 and Li3FeN2, which belong to the Li3N structure. Both have good reversibility and high specific capacity, and their main properties are shown in Table 1. 2.2 Metal oxide carbon, as the negative electrode of lithium-ion batteries, forms a passivation layer on the carbon surface in organic electrolyte solutions that allows electrons and lithium ions to freely pass through, ensuring good cycling performance of the carbon electrode. However, it can also cause serious irreversible capacity loss during the first charge and discharge, and sometimes even lead to structural changes and poor electrical contact inside the carbon electrode. In addition, battery failure or safety issues may occur due to the decomposition of the protective layer at high temperatures. Therefore, while studying carbon anodes, the search for other negative electrode materials with similar potentials to Li+/Li pairs has been highly valued, such as SnO, WO2, MoO2, VO2, TiO2, LixFe2O3, Li4Mn2O12, Li4Ti5O12, etc., among which SnO materials are the focus of research. This is due to the advantages of tin based oxide lithium storage materials, such as high capacity density, cleanliness and pollution-free, wide source of raw materials, and low price. In 1997, Yoshioldota et al. reported an amorphous tin oxide based lithium storage material with a reversible discharge capacity of 600mAh · g-1, low insertion and extraction lithium potentials, stable electrode structure, and good cycling performance. Nam et al. used electron beam deposition of 1 μ m thick SnO as the negative electrode material for thin-film lithium-ion batteries, and after 100 cycles of charging and discharging, the displayed capacity exceeded 300mAh · g-1. SCNam et al. prepared crystalline SnO2 thin films using chemical vapor deposition method. Cyclic voltammetry experiments showed that there was an irreversible capacity in the first cycle, which was believed to be caused by the formation of amorphous Li2O and metallic tin. In subsequent cycles, metallic tin acted as a reversible electrode with a capacity of up to 500mAh · g-1 and exhibited good cycling performance. 2.3 The electrode reaction between lithium intermetallic compound and metal oxide is different from the insertion extraction reaction of lithium in carbon materials. The former is the alloying and dealloying process of Li with other metals. When using metal oxide as the negative electrode, the Li2O formed for the first time during the charging process can serve as a structural support in the negative electrode, but it also has a large irreversible capacity. So, in order to reduce the irreversible capacity of the electrode while maintaining the stability of the negative electrode structure, intermetallic compounds can be used as the negative electrode of lithium-ion batteries. However, it should also be noted that the reversible generation and decomposition of Li-M alloys are accompanied by significant volume changes, leading to alloy splitting. The solution is to prepare active materials with extremely fine particles to prevent the formation of large atomic clusters, and to use sliding or non active composite alloys. Inert metals that do not react with Li serve as the matrix and conductive components to accommodate the alloy components. In this regard, previous researchers have conducted extensive research. MaoOu et al. [4-6] synthesized Sn-Fe-I powder; M. M. Thackeray and D Larcher et al. investigated the lithium storage properties of Cu Sn alloys; J. O. Besenhard synthesized polycrystalline Sn Sb alloy using solid-phase method and nanocrystalline Sn Sb alloy using electrolytic method; J.Yangt、 Li Hong et al. prepared nano Sn Sb alloy using NaBH4 and Zn powder as reducing agents in aqueous solution; C. M. Ehrilich et al. synthesized Sn Ni alloy using the MM method. Fang · L et al. studied amorphous Sn Ca alloys. It was found that the initial lithium storage capacity of these alloys was relatively large, but their cycling performance was not ideal, as shown in Table 2. To achieve good cycling performance, its capacity needs to be reduced significantly (around 200mAh/g), and the cycling range is relatively narrow, which limits its application to a certain extent. HirokilS et al. synthesized Mg2.0Ce using mechanical alloying (MA) method. At 25 hours, the crystallinity of MA was found to be 90%, and the initial capacity was 320mAh/g. At 100h, the crystallinity of MA is approximately 0, and the initial capacity is 25mAh/g, but the cycling performance is good. HansuK et al. studied Mg Si alloys and found that the negative electrode capacity of Mg2Si was about 1370mAh/g, with a flat voltage curve. However, the large volume change caused electrode detachment. HansuK et al. also studied Mg-N alloys and found that Mg75N25 reacted with Li at room temperature, resulting in significantly improved cycling performance compared to pure Mg. Cao et al. prepared Zn4Sb3 (- C7) by vacuum melting method, with an initial capacity of 581mAh/g. After 10 cycles, the capacity is 402mAh/g. Huang S. M et al. prepared SiAg alloy. The SiAg electrode polished for 50 hours showed good cycling performance and minimal capacity loss, with a reversible capacity of 280mAh/g after more than 50 cycles. ZhangLT et al. developed CoFe3Sb12 with an initial reversible capacity of 490mAh/g. After 10 cycles, the reversible capacity remained above 240mAh/g. There have also been many reports on the research of Al in recent years. According to the A1 Li binary phase diagram, Al and Li can form three possible intermetallic compounds: A1Li, Al2Li3, and Al4Li9. So, the theoretical maximum lithium capacity of Al electrode is 2.25 Li atoms per can atom on average, which corresponds to the Li rich phase Al4Li9. Its theoretical specific capacity is 2234mAh/g, much higher than the theoretical specific capacity of 372mAh/g of graphite. However, when using pure Al as the negative electrode, there are also issues of large capacity loss and poor cycling performance. Hamon et al. believe that pure A1 has a specific capacity of over 1000mAh/g as the negative electrode of lithium-ion batteries, due to the formation of amorphous Li Al alloy between lithium ions and Al during insertion and extraction processes. And its poor recyclability is caused by the significant volume change of the Al electrode during the charge discharge cycle. Meanwhile, Hamon et al. also found that the thinner the A1 foil sample, the smaller the volume change of the electrode after charge and discharge cycles, resulting in better cycling performance. This also confirms that in order to solve the problem of poor electrode cycling caused by the huge volume changes accompanying the reversible generation and decomposition of Li-M alloys, we can prepare extremely fine particle active materials or ultra-thin thin film materials. In addition, we can also use inert metal elements added to elemental metals that can react with Li to prepare some active or inactive composite alloys to solve this problem. Machill et al. [21-22] can improve the cycling performance of AI electrodes by adding some metal elements that are soluble in Al or can form intermetallic compounds with Al, such as Ni, Cu, Mg, etc., to improve the diffusion rate of Li during the embedding process in the negative electrode, thereby enhancing the cycling performance of A1 electrodes. Although adding other metal elements to Al electrodes can reduce their specific capacity and energy density, the resulting improvement in cycling performance can compensate for this deficiency. Therefore, Al based intermetallic compounds have broad development prospects as negative electrode materials for lithium-ion batteries. Conclusion: In recent years, practical research on negative electrode materials for lithium-ion batteries has mainly focused on how to improve mass specific capacity and volume specific capacity, first charge discharge efficiency, cycle performance, and cost reduction. Graphite negative electrode materials have been successfully commercialized, but there are still some weaknesses that are difficult to overcome. This is because the carbon negative electrode forms a passivation layer (SEI layer) in organic electrolytes. Although this layer can transfer electrons and lithium ions, it can cause irreversible loss of initial capacity; Moreover, the potential of the carbon electrode is very close to that of metallic lithium. When the battery is overcharged, metallic lithium is prone to precipitate on the surface of the carbon electrode, which may form lithium dendrites and cause a short circuit; Secondly, at high temperatures, the protective layer on the carbon negative electrode may decompose, leading to battery ignition; In addition, the performance of carbon electrodes is greatly affected by the preparation process. Given the above situation, finding non carbon negative electrode materials with better performance is still an important topic in the research of lithium-ion batteries. In recent years, many researchers have reported their achievements in studying non carbon negative electrode materials, especially in the field of intermetallic compounds. Their research results indicate that metal based compounds have a slight decrease in specific capacity compared to pure metal negative electrode materials, but their cycling performance is significantly improved. The reason is that adding other metal elements to the active metal can significantly reduce the volume change of the metal negative electrode during cycling, and the introduced other inert metals can also serve as the skeleton material to carry some components. Currently, research on lithiation of metal compounds mainly focuses on Sn based intermetallic compounds. This is because Sn can react with L1 to form various intermetallic compounds, and their lithium insertion capacity is relatively high. Therefore, Sn based intermetallic compounds can not only achieve good cycling performance, but also their specific capacity will not decrease significantly. Al can also form various intermetallic compounds, but current research is relatively limited.

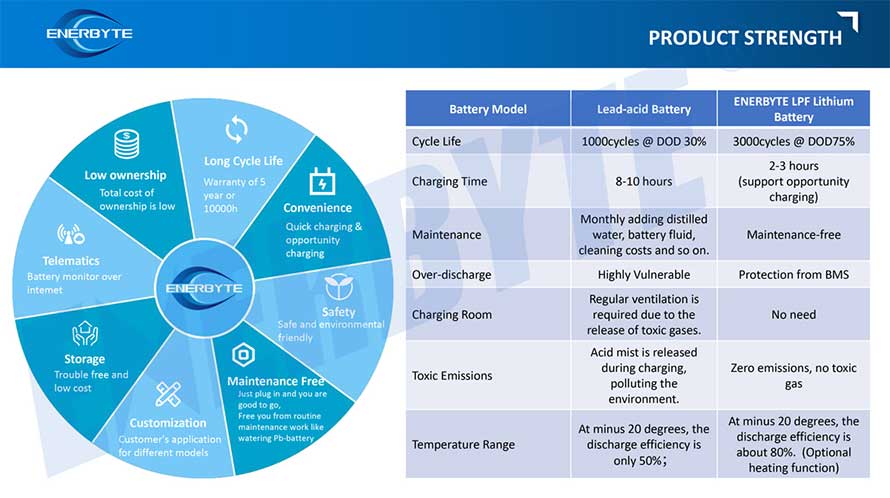
Lithium Batteries ,Ensure Quality
Our lithium battery production line has a complete and scientific quality management system
Ensure the product quality of lithium batteries

Years of experience in producing lithium batteries
Focus on the production of lithium batteries

WE PROMISE TO MAKE EVERY LITHIUM BATTERY WELL
We have a comprehensive explanation of lithium batteries

QUALIFICATION CERTIFICATE
THE QUALITY OF COMPLIANCE PROVIDES GUARANTEE FOR CUSTOMERS
MULTIPLE QUALIFICATION CERTIFICATES TO ENSURE STABLE PRODUCT QUALITY
Providing customers with professional and assured products is the guarantee of our continuous progress.



Applicable brands of our products


 Service hotline
Service hotline